Search results
Search for "ionic liquid" in Full Text gives 37 result(s) in Beilstein Journal of Nanotechnology.
Application of nanoarchitectonics in moist-electric generation
Beilstein J. Nanotechnol. 2022, 13, 1185–1200, doi:10.3762/bjnano.13.99

- gradient structure can be constructed relative to any direction in space to meet the needs. Moisture-electric conversion devices in nanofluids have also been recently reported. An ionic liquid film of Omim+ Cl− was selected as the ion-exchange layer [88]. In a moisture-gradient environment (Figure 9) on
- both sides of the ionic liquid layer a humidity difference was constructed. Ions are driven by the ion concentration difference and then pass through the membrane by selective ion diffusion. The ion-selective membrane is designed to hinder specific ions from passing through the membrane such that a
- velocity) for three droplets of different chloride and sodium salts. Figure 2c–f are from [8] and were reprinted by permission from Springer Nature from the journal Nature Nanotechnology (“Generating electricity by moving a droplet of ionic liquid along graphene“ by J. Yin; X. Li; J. Yu; Z. Zhang; J. Zhou
Sputtering onto liquids: a critical review
Beilstein J. Nanotechnol. 2022, 13, 10–53, doi:10.3762/bjnano.13.2
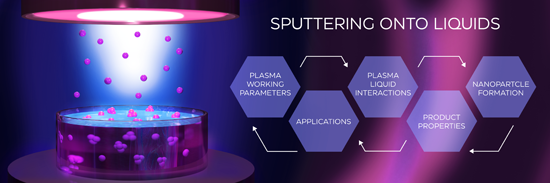
- [11]. Afterward, Torimoto et al. used a sputter coater for the synthesis of small gold (Au) NPs by sputtering an Au target onto a thin layer of an ionic liquid (IL) [12]. This paper [12] published in 2006 initiated a series of similar works in several labs which, were focused on the deposition of
Electrical, electrochemical and structural studies of a chlorine-derived ionic liquid-based polymer gel electrolyte
Beilstein J. Nanotechnol. 2021, 12, 1252–1261, doi:10.3762/bjnano.12.92

- Technology, Shirgaon, Virar East, Maharastra, 401305, India Department of Physics, School of Physical Sciences, Mahatma Gandhi Central University, Bihar-845401, India 10.3762/bjnano.12.92 Abstract In the present article, an ionic liquid-based polymer gel electrolyte was synthesized by using poly(vinylidene
- electrolyte film which contains 30 wt % of the ionic liquid. The optimized films have good potential to be used as electrolyte materials for energy storage applications. Keywords: ionic liquid; polymer gel electrolytes; solution casting technique; transference number; Introduction For the past two decades
- ionic liquid [BDiMIM][Cl], the host polymer PVdF-HFP, and the salt magnesium perchlorate (Mg(ClO4)2) were used to prepare polymer electrolyte films. The synthesized films were characterized by different microscopic and electrochemical techniques. Results and Discussion Structural characterization The
Nickel nanoparticle-decorated reduced graphene oxide/WO3 nanocomposite – a promising candidate for gas sensing
Beilstein J. Nanotechnol. 2021, 12, 343–353, doi:10.3762/bjnano.12.28

- achieved [47]. Thermally reduced graphene oxide was tested before with different metals in ionic liquids [48][49]. The decoration of nanoparticles on rGO can be achieved in situ or by mixing previously prepared solutions [50]. Here, we chose the ionic liquid [BMIm][NTf2] for an in situ microwave
- molecular sieves in a nitrogen atmosphere. Final water contents measured by coulometric Karl Fischer titration (ECH/ANALYTIK JENA AQUA 40.00) did not exceed 10 ppm. Bis(1,5-cyclooctadiene)nickel(0) (Ni(COD)2) was purchased from ABCR, stored at −4 °C and used without further purification. The ionic liquid
- was dried at 100 °C using a turbo molecular pump at 5 × 10−7 mbar for several days. Preparation of Ni@rGO in ionic liquid Nickel nanoparticles on rGO (Ni@rGO) were prepared in septum-sealed 10 mL microwave vessels (CEM GmbH, Germany) in a CEM Discover microwave under argon atmosphere. Ni(COD)2 (49.2
Bio-imaging with the helium-ion microscope: A review
Beilstein J. Nanotechnol. 2021, 12, 1–23, doi:10.3762/bjnano.12.1

- eliminates the need for coating biological samples with conductive materials to obtain high-resolution information. In fact, this is often cited as one of the main benefits of using HIM and has been reported in a number of publications focusing on biological samples [11][62][65]. Ionic liquid preparation An
- a typical electron microscope and exhibit conductive properties. This means that samples can be immersed in an ionic liquid, for 10–600 s, blotted, loaded onto a sample holder, and then imaged [68]. Compared to other preparation techniques, the preparation time using ionic liquids is extremely short
- prepared with an ionic liquid (Figure 4B). Though the HMDS-prepared sample clearly shows a high density of individual bacteria, the ionic liquid treatment appears to have maintained the EPS and is perhaps a better representation of the true biofilm. Nevertheless, this study suggests that multiple
Gas-sensing features of nanostructured tellurium thin films
Beilstein J. Nanotechnol. 2020, 11, 1010–1018, doi:10.3762/bjnano.11.85

- galvanic displacement of sacrificial cobalt nanowires were employed [15]. Lastly, to grow one-dimensional nanostructures, either template-free electrodeposition of Te, from an ionic liquid binary mixture [16], or thermal evaporation in a furnace under argon gas flow [17] were strategies utilized. The
Nickel nanoparticles supported on a covalent triazine framework as electrocatalyst for oxygen evolution reaction and oxygen reduction reactions
Beilstein J. Nanotechnol. 2020, 11, 770–781, doi:10.3762/bjnano.11.62

- nanoparticles supported on CTF-1 in the ionic liquid (IL) [BMIm][NTf2] using a microwave-assisted synthesis. The obtained material Ni/CTF-1 was investigated as a catalyst for electrochemical OER and ORR for the first time and showed a superior OER performance compared to commercial RuO2 under alkaline
- a fast and efficient microwave synthesis within 10 min from Ni(COD)2 and the CTF substrate in an ionic liquid. The choice of the CTF substrate enables the control over nitrogen doping by selecting appropriate aromatic nitriles as monomers [32][37][40]. Also, it has been proven that the use of CTFs
- ABCR. All materials were used without further purification. 1-Methylimidazole (>99%) was obtained from Sigma Aldrich, purified via fractional distillation and dried over molecular sieves for several days. Water was purified using the Millipore® system. The ionic liquid (IL) 1-butyl-3-methylimidazolium
Review of advanced sensor devices employing nanoarchitectonics concepts
Beilstein J. Nanotechnol. 2019, 10, 2014–2030, doi:10.3762/bjnano.10.198

- , vinylidenefluoride-tetrafluoroethylene-hexafluoropropylene, and an ionic liquid, 1-butyl-3-methylimidazolium bis(trifluoromethanesulphonyl)imide, through an electrospinning process. The prepared sensor can only sense normal pressure without significant disturbance up to a bending radius of 80 µm. This sensor system
Synthesis of nickel/gallium nanoalloys using a dual-source approach in 1-alkyl-3-methylimidazole ionic liquids
Beilstein J. Nanotechnol. 2019, 10, 1754–1767, doi:10.3762/bjnano.10.171

- = 1,5-cyclooctadiene) and GaCp* (Cp* = pentamethylcyclopentadienyl) in the ionic liquid [BMIm][NTf2] selectively yields small intermetallic Ni/Ga nanocrystals of 5 ± 1 nm as derived from transmission electron microscopy (TEM) and high-angle annular dark-field scanning transmission electron microscopy
- oxidation state +1, was reported to form phase-pure NiGa and Ni3Ga nanoparticles with Ni(COD)2 in the ionic liquid [BMIm][BF4] under microwave-induced pyrolysis at 230 °C [30]. GaCp* is reported to be thermally stable in organic solvents in the absence of hydrogen to up to 300 °C [43]. In imidazolium-based
- ionic liquids decomposition of GaCp* is possible at temperatures below 300 °C with the aid of transition metals. Reactions of transition-metal complexes are reported to show H/D activation/exchange reactions at the C2 imidazolium carbon atom of the ionic liquid cation. The generated N-heterocyclic
Upcycling of polyurethane waste by mechanochemistry: synthesis of N-doped porous carbon materials for supercapacitor applications
Beilstein J. Nanotechnol. 2019, 10, 1618–1627, doi:10.3762/bjnano.10.157

- aqueous Li2SO4, organic TEA-BF4 in acetonitrile, and an ionic liquid EMIM-BF4 electrolyte. Results and Discussion Characterization and mechanochemical treatment of PU Polyurethane is a polymer formed by polyaddition of diisocyanates R1(–NCO)2 with polyols R2(–OH)n. It is characterized by the resulting
- ). This can be associated with the higher volume ratio of mesopores to micropores, since mesopores are acting as transport pores enabling a fast electrolyte ion mobility [73][74]. Interestingly, the rate capability for this material in organic and ionic liquid electrolytes, however, is worse compared to
- Li2SO4), organic (1 M TEA-BF4 in ACN) and ionic liquid (EMIM-BF4) electrolytes calculated from galvanostatic charge–discharge measurements at different specific currents. Sample code and amounts of PU, urea and K2CO3. Supporting Information Supporting Information File 254: Materials and methods
Synthesis of P- and N-doped carbon catalysts for the oxygen reduction reaction via controlled phosphoric acid treatment of folic acid
Beilstein J. Nanotechnol. 2019, 10, 1497–1510, doi:10.3762/bjnano.10.148

- results of previous studies on PN-doped carbon materials, which reported the facile formation of pyridinic N upon P-doping. For example, Gao et al. prepared a PN-doped carbon material by carbonization of an ionic liquid synthesized from N-methylimidazole and PA and reported the selective formation of
Alloyed Pt3M (M = Co, Ni) nanoparticles supported on S- and N-doped carbon nanotubes for the oxygen reduction reaction
Beilstein J. Nanotechnol. 2019, 10, 1251–1269, doi:10.3762/bjnano.10.125

- ) alloyed nanoparticles that have a very homogeneous size distribution (in spite of the high metal loading of ≈40 wt % Pt), using an ionic liquid as a stabilizer. The electrochemical surface area, the activity for the oxygen reduction reaction and the amount of H2O2 generated during the oxygen reduction
- nanoparticles, on the preparation of the catalytic layer, and on the electrocatalytic performance in the ORR. On N-CNT supports, the specific activity followed the expected order Pt3Co > Pt3Ni, whereas on the annealed N-CNT support, the order was reversed. Keywords: carbon nanotubes; cobalt; ionic liquid
- prepared using the transmetalation method described by Kristian et al. [23]. The redox couple used for the transmetalation is Co2+(II)/Co(0) (E° = −0.77 V/RHE) and PtCl42−/Pt(0) (E° = 0.67 V/RHE) for Pt3Co NPs. In our procedure, the ammonium salt used as a stabilizer has been replaced by an ionic liquid in
Porous N- and S-doped carbon–carbon composite electrodes by soft-templating for redox flow batteries
Beilstein J. Nanotechnol. 2019, 10, 1131–1139, doi:10.3762/bjnano.10.113

- possible to scale-up the method to industrial standards [21]. In earlier studies we reported on a salt-templating method, where we embedded a PAN-based carbon felt into a eutectic mixture containing zinc chloride and sodium chloride mixed with an ionic liquid as the carbon source [22]. Herein, we present
Synthesis and characterization of quaternary La(Sr)S–TaS2 misfit-layered nanotubes
Beilstein J. Nanotechnol. 2019, 10, 1112–1124, doi:10.3762/bjnano.10.111

- resonators [11][12][13]. Using ionic liquid gating, ambipolar p–n junctions led to high-performance light-emitting diodes (LEDs) and photovoltaic devices [14]. Most interesting, however, was the demonstration of quasi-1D superconductivity, which reflected the non-centrosymmetric structure of the chiral WS2
Deposition of metal particles onto semiconductor nanorods using an ionic liquid
Beilstein J. Nanotechnol. 2019, 10, 718–724, doi:10.3762/bjnano.10.71

- an ionic liquid, and the effect this has on catalytic performance. Platinum, gold, and silver nanoparticles were deposited onto CdSe@CdS (core@shell) nanorods from metal salts in an ionic liquid (1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide) without additional surfactants or reducing
- agents. Photocatalytic dye degradation experiments showed that catalysts with platinum particles deposited using the ionic liquid out-performed similar materials synthesized using organic solvents and ligands. We concluded that metal particles can be deposited onto well-defined semiconductor nanorods
- using the methods reported here. Keywords: catalyst; ionic liquid; methylene blue; platinum; semiconductor nanorod; Introduction Core@shell semiconductor nanorods with attached noble metal particles have been widely studied as photocatalysts, and any improvement on the synthesis of these materials has
Electrolyte tuning in dye-sensitized solar cells with N-heterocyclic carbene (NHC) iron(II) sensitizers
Beilstein J. Nanotechnol. 2018, 9, 3069–3078, doi:10.3762/bjnano.9.285

- reported N-heterocyclic carbene iron(II) dye in the presence of chenodeoxycholic acid co-adsorbant, can be considerably improved by altering the composition of the electrolyte while retaining an I−/I3− redox shuttle. Critical factors are the solvent, presence of ionic liquid, and the use of the additives 1
- -methylbenzimidazole (MBI) and 4-tert-butylpyridine (TBP). For the electrolyte solvent, 3-methoxypropionitrile (MPN) is preferable to acetonitrile, leading to a higher short-circuit current density (JSC) with little change in the open-circuit voltage (VOC). For electrolytes containing MPN, an ionic liquid and MBI (0.5
- M), DSC performance depended on the ionic liquid with 1-ethyl-3-methylimidazolium hexafluoridophosphate (EMIMPF) > 1,2-dimethyl-3-propylimidazolium iodide (DMPII) > 1-butyl-3-methylimidazolium iodide (BMII) ≈ 1-butyl-3-methylimidazolium hexafluoridophosphate (BMIMPF). Omitting the MBI leads to a
Nanocellulose: Recent advances and its prospects in environmental remediation
Beilstein J. Nanotechnol. 2018, 9, 2479–2498, doi:10.3762/bjnano.9.232

- used to isolate cellulose fibres to generate nanocellulose. Existing chemical pretreatment techniques include acid hydrolysis [69], carboxylation [70], carboxymethylation [71], quaternization [72], sulphonation [73], ionic liquid [74], and solvent-assisted pretreatments [75]. Acid hydrolysis is a
- hydrolysis has become a popular alternative method for nanocellulose extraction. The extraction of nanocellulose using ionic liquids, such as 1-butyl-2-methyllimidazolium hydrogen sulphate (BmimHSO4), has been considered as a green approach for its economical process (due to high recovery of ionic liquid for
- magnetic field. As a result, magnetic nanomaterials have drawn increasing attention. Nanocellulose incorporated with other magnetic nanomaterials is presented as an excellent composite adsorbent with magnetic properties. For example, a core–shell cellulose magnetite (Fe3O4) polymeric ionic liquid magnetic
Synthesis of rare-earth metal and rare-earth metal-fluoride nanoparticles in ionic liquids and propylene carbonate
Beilstein J. Nanotechnol. 2018, 9, 1881–1894, doi:10.3762/bjnano.9.180
- [20]. Herein, we report the use of rare-earth amidinates RE(amd)3 with RE = Pr(III), Gd(III), Er(III) and of tris(2,2,6,6-tetramethyl-3,5-heptanedionato)europium(III) (Eu(dpm)3) as precursors for rare-earth metal-fluoride nanoparticles (REF3-NPs) by microwave-assisted thermal syntheses in the ionic
- liquid (IL) 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIm][BF4]) with the reactive BF4 anion as a fluoride source (Scheme 1). For the synthesis of EuF3-NPs, the precursor Eu(dpm)3 was used, since a europium amidinate, Eu(amd)3 is not yet known in the literature and therefore not available. In
A simple extension of the commonly used fitting equation for oscillatory structural forces in case of silica nanoparticle suspensions
Beilstein J. Nanotechnol. 2018, 9, 1095–1107, doi:10.3762/bjnano.9.101

- Moazzami-Gudarzi et al. [55][56], in case of strong polyelectrolytes between two micrometer-sized silica spheres and the group of Perkin, namely Alexander M. Smith et al. [57] and Samuel W. Coles et al. [58], for mixtures of an ionic liquid with a polar solvent using SFA. Both attributed the additional
Fabrication and photoactivity of ionic liquid–TiO2 structures for efficient visible-light-induced photocatalytic decomposition of organic pollutants in aqueous phase
Beilstein J. Nanotechnol. 2018, 9, 580–590, doi:10.3762/bjnano.9.54

- , Chemical Faculty, Department of Chemical Technology, Narutowicza 11/12, 80-233 Gdansk, Poland, Institute of Physical Chemistry, Polish Academy of Sciences, 01-224 Warsaw, Poland 10.3762/bjnano.9.54 Abstract To investigate the effect of the ionic liquid (IL) chain length on the surface properties and
- nm). The highest photoefficiency (four times higher than pristine TiO2) was observed for the TiO2 sample obtained in the presence of [TDMIM][Cl] for a IL to TiO2 precursor molar ratio of 1:3. It was revealed that interactions between the ions of the ionic liquid and the surface of the growing
- photocatalysts [12][13][36][37][38]. Interestingly, in all mentioned publications [12][13][36][37][38] the same ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]), was used. However, the proposed mechanisms were different. The photoactivity enhancement under visible-light irradiation was
Dielectric properties of a bisimidazolium salt with dodecyl sulfate anion doped with carbon nanotubes
Beilstein J. Nanotechnol. 2018, 9, 164–174, doi:10.3762/bjnano.9.19

- Institute of Materials Physics, POBox MG 07, 077125 Magurele, Romania 10.3762/bjnano.9.19 Abstract A new bisimidazolium salt with dodecyl sulfate as counterion has been designed and prepared. This salt shows a SmA phase that is stable at room temperature. The new ionic liquid crystal (ILC) was
- low frequencies confirm the presence of EP. Keywords: activation energy; carbon nanotubes; dielectric spectroscopy; ionic liquid crystal; relaxation time; Introduction Ionic liquid crystals (ILCs) represent a very appealing class of materials that has found various recent applications in dye
- -sensitized solar cells, battery materials, electrochemical sensors or energy storage devices. Their interesting properties result from the combination of liquid crystal (LC) and ionic liquid (IL) properties. The recent progress and development in the field of ILCs were reviewed in several publications [1][2
Synthesis of metal-fluoride nanoparticles supported on thermally reduced graphite oxide
Beilstein J. Nanotechnol. 2017, 8, 2474–2483, doi:10.3762/bjnano.8.247

- (AMD)n] with M = Fe(II), Co(II), Pr(III), and tris(2,2,6,6-tetramethyl-3,5-heptanedionato)europium, Eu(dpm)3, in the presence of TRGO in the ionic liquid (IL) 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIm][BF4]). The crystalline phases of the metal fluorides synthesized in [BMIm][BF4] were
- Suzuki–Miyaura coupling reaction [14]. In 2011, metal carbonyls in dispersion with TRGO and ionic liquid (IL) were exposed to short low-energy microwave irradiation. The resulting Ru@TRGO and Rh@TRGO particles had high catalytic hydrogenation activity [12]. Metallic nanoparticles on graphene have
- functionalities. Results and Discussion Transition-metal amidinates [M(AMD)n; M = Fe(II), Co(II), Pr(III)] as well as Eu(dpm)3 were dissolved or suspended under nitrogen atmosphere in the dried and deoxygenated ionic liquid together with the selected type of thermally reduced graphene oxide (TRGO). Complete
Two-dimensional carbon-based nanocomposites for photocatalytic energy generation and environmental remediation applications
Beilstein J. Nanotechnol. 2017, 8, 1571–1600, doi:10.3762/bjnano.8.159

- the preparation of g-C3N4 based nanocomposites, which includes molecular self-assembly [93], microwave assisted heating [38], molten salt synthesis [94] and ionic liquid strategy [95]. 2D carbon-based nanocomposites as photocatalysts 2D graphene-based photocatalysts for energy generation
First examples of organosilica-based ionogels: synthesis and electrochemical behavior
Beilstein J. Nanotechnol. 2017, 8, 736–751, doi:10.3762/bjnano.8.77

- transport. A new preparation process using an organic linker, bis(3-(trimethoxysilyl)propyl)amine (BTMSPA), yields stable organosilica matrix materials. The second ionogel component, the ionic liquid 1-methyl-3-(4-sulfobutyl)imidazolium 4-methylbenzenesulfonate, [BmimSO3H][PTS], can easily be prepared with
- near-quantitative yields. [BmimSO3H][PTS] is the proton conducting species in the ionogel. By combining the stable organosilica matrix with the sulfonated ionic liquid, mechanically stable, and highly conductive ionogels with application potential in sensors or fuel cells can be prepared. Keywords
- addressed in polymer-ionic liquid pairing [56]. To further evaluate the molecular processes occurring in the IGs, we have performed computational studies on the pure IL [BmimSO3H][PTS]. Figure 15 shows that there are three states of the SO3H-proton in its intramolecular O–H bond (the bond is described in
Formation and shape-control of hierarchical cobalt nanostructures using quaternary ammonium salts in aqueous media
Beilstein J. Nanotechnol. 2017, 8, 494–505, doi:10.3762/bjnano.8.53

- obtain cobalt nanostructures of desired size and shape such as spherical nanoparticles [14][15] synthesized by high-temperature chemical reduction while controlling the pH value, cobalt–polymer composite tubes [16] formed by using alumina templates, cobalt cubes [17] produced in imidazolium ionic liquid














































































































































































































































































